TOSB – Otomotiv Yan Sanayi İhtisas OSB 1. Cad. 15 Yol No:1
41420 Çayırova / Kocaeli / TÜRKİYE
Phone: +90 262 658 29 14
Fax: +90 262 658 18 19
Email: info@sistemteknik.com
5 July 2023
Ammonia is preferred in various industries such as fertilizer and chemical cleaning agents production, as well as cooling and industrial furnace industries. Ammonia contains 1.7 times more hydrogen per unit volume than liquid hydrogen. In recent years, with the emergence of fuel cell technology, it is known that ammonia is a promising fluid in terms of energy production due to its high energy density. Ammonia decomposers are used as protective or process atmospheres in the industrial furnace sector.
The main processes involved are listed below:
The primary function of ammonia decomposers is to break down ammonia into hydrogen and nitrogen. The main reaction occurring in these decomposers is given below:
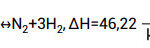
From the formula, it can be observed that for 2 moles of gas input, there are 4 moles of gas output (the outgoing gas stream is referred to as synthesis gas). Synthesis gas theoretically consists of 75% hydrogen and 25% nitrogen. The rate of ammonia decomposition depends on factors such as pressure, temperature, feed flow rate, and the type and shape of catalyst used. This publication focuses on examining the parameters that affect the decomposition rate.
The theoretical limit for the lowest operating temperature in ammonia decomposers is determined by the chemical equilibrium of the decomposition reaction. Figure 1 illustrates the decomposition rates of ammonia fed to the decomposer at different pressures. As seen from the figure, as the pressure of ammonia fed to the decomposer increases, the reaction temperature must also be increased to enhance the decomposition efficiency. It can be observed from Figure 1 that reaching temperatures around 430°C is sufficient for ammonia decomposition at atmospheric pressure. However, this temperature range applies to large reactors with excellent catalysts. Due to the small size of ammonia decomposers and the less-than-perfect catalyst efficiency, the temperature range needs to be increased.

Catalysts are used in ammonia decomposers to achieve decomposition at lower reaction temperatures. Nickel-based catalysts are the most preferred type in the industry. The catalytic activity, and thus the conversion at a constant temperature and pressure, is enhanced by adding elements and compounds such as platinum, palladium, lanthanum oxide, and ruthenium to the nickel-based catalyst. Previous technical studies have been conducted to increase the decomposition rate by increasing the reactor temperature as the ammonia flow rate increases. The findings are presented in Figure 2. As shown in Figure 2, the greatest contribution to the conversion rate is achieved by incorporating ruthenium into the catalyst. One noteworthy result is that a nickel-based catalyst with added ruthenium was indicated to achieve a nearly 100% conversion rate. Figure 3 illustrates this situation with a graph. The graph shows a temperature difference of 100 K between the polynomial and ruthenium-doped nickel-based catalyst for ammonia feed rates between 0 and 100 l/h. Due to the scarcity of ruthenium and the resulting higher cost of the catalyst, higher reactor temperatures are preferred in the industry to achieve the same efficiency as ruthenium-doped nickel-based catalysts.

It has been previously studied that the reactor temperature needs to be increased to maximize the conversion rate as the ammonia flow rate increases. The findings are presented in Figure 2. As shown in Figure 2, the greatest contribution to the conversion rate is achieved by incorporating ruthenium into the catalyst. One noteworthy result is that a nickel-based catalyst with added ruthenium was indicated to achieve a nearly 100% conversion rate. Figure 3 illustrates this situation with a graph. The graph shows a temperature difference of 100 K between the polynomial and ruthenium-doped nickel-based catalyst for ammonia feed rates between 0 and 100 l/h. One of the significant findings is that the ammonia flow rate linearly increases the conversion rate when fed at higher flow rates.